(CLR 125): Auger Emitter (125I)
A next-generation investigational phospholipid ether (PLE) conjugate designed for highly localized radiation delivery through Auger electron emission.
A next-generation investigational phospholipid ether (PLE) conjugate designed for highly localized radiation delivery through Auger electron emission.
CLR 125 is an investigational radioconjugate designed to deliver iodine-125 directly into tumor cell nucleus using Cellectar’s proprietary phospholipid ether (PLE) platform.
Iodine-125 emits Auger electrons—high-energy, short-range particles capable of inducing clustered/larger DNA double-strand breaks within targeted tumor cells while essentially eliminating any dose to surrounding healthy tissue.
Auger emitters release multiple low-energy electrons that travel a few nanometers within cells, creating dense, localized energy deposits that disrupt tumor DNA.
CLR 125’s selective uptake enables potent, targeted damage with limited off target effects.
| Name | Composition | Primary Mechanism of Cell Death | Penetrating Power (Emission Distance) | Relative Biologic Effect |
|---|---|---|---|---|
| Alpha Particles |
2 protons |
Double strand |
50 – 100um |
~5 |
| Beta Particles |
1 electron |
Single strand |
12mm |
1 |
| Auger Electrons |
Multiple electrons |
Double strand |
2 – 500nm |
1 – 5 |
Properties of different emitters provide enhanced outcomes
In preclinical studies, CLR 125 showed selective tumor uptake and statistically significant activity in animal models of triple-negative breast cancer (TNBC), with no observed end-organ or hematologic toxicity at evaluated doses.
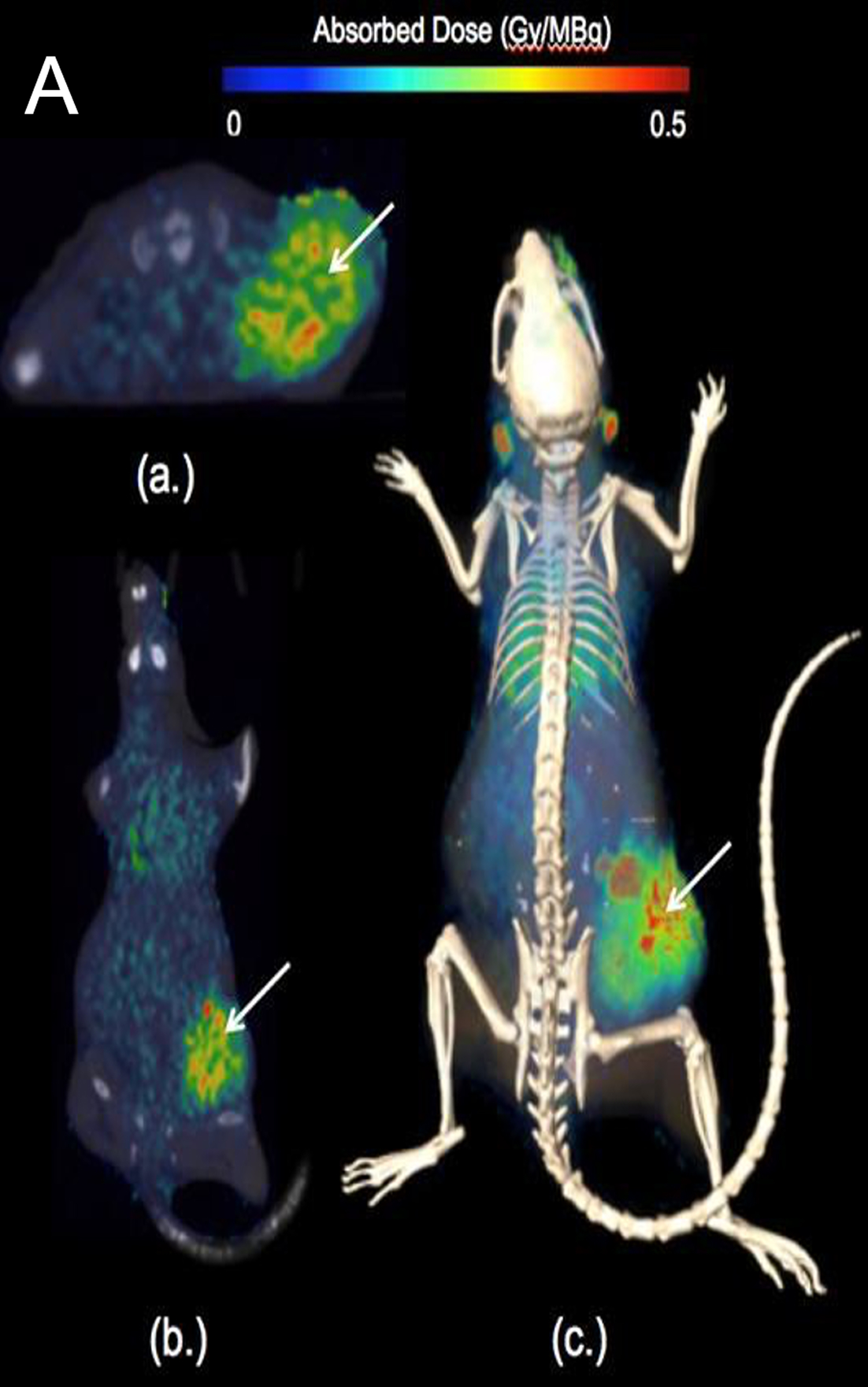


The figure presents CLR 125 preclinical results in triple-negative breast cancer (MDA-MB-231). Heat map image shows a color-scaled PET/CT image with strong tumor uptake. Bar chart is indicating the highest absorbed dose in tumor tissue compared with normal organs such as liver, kidneys, and brain. Line graph demonstrates tumor growth inhibition at 1.2 mCi and 2.0 mCi compared with control. These data demonstrate selective tumor localization, significant dose-dependent growth inhibition, and absence of end-organ or hematologic toxicity.
Validated intracellular delivery with CLR 121125
CLR 125 is currently being studied in a Phase 1b imaging and therapy trial for patients with relapsed or refractory triple-negative breast cancer (TNBC). The study, a multi-center study including the Mayo Clinic Network, aims to determine the recommended Phase 2 dose and evaluate safety and tolerability.
CLR 125 represents the continued evolution of Cellectar’s PLE platform, extending its precision-targeting approach across multiple isotope classes—beta (I-131), alpha (Ac-225), and Auger (I-125).
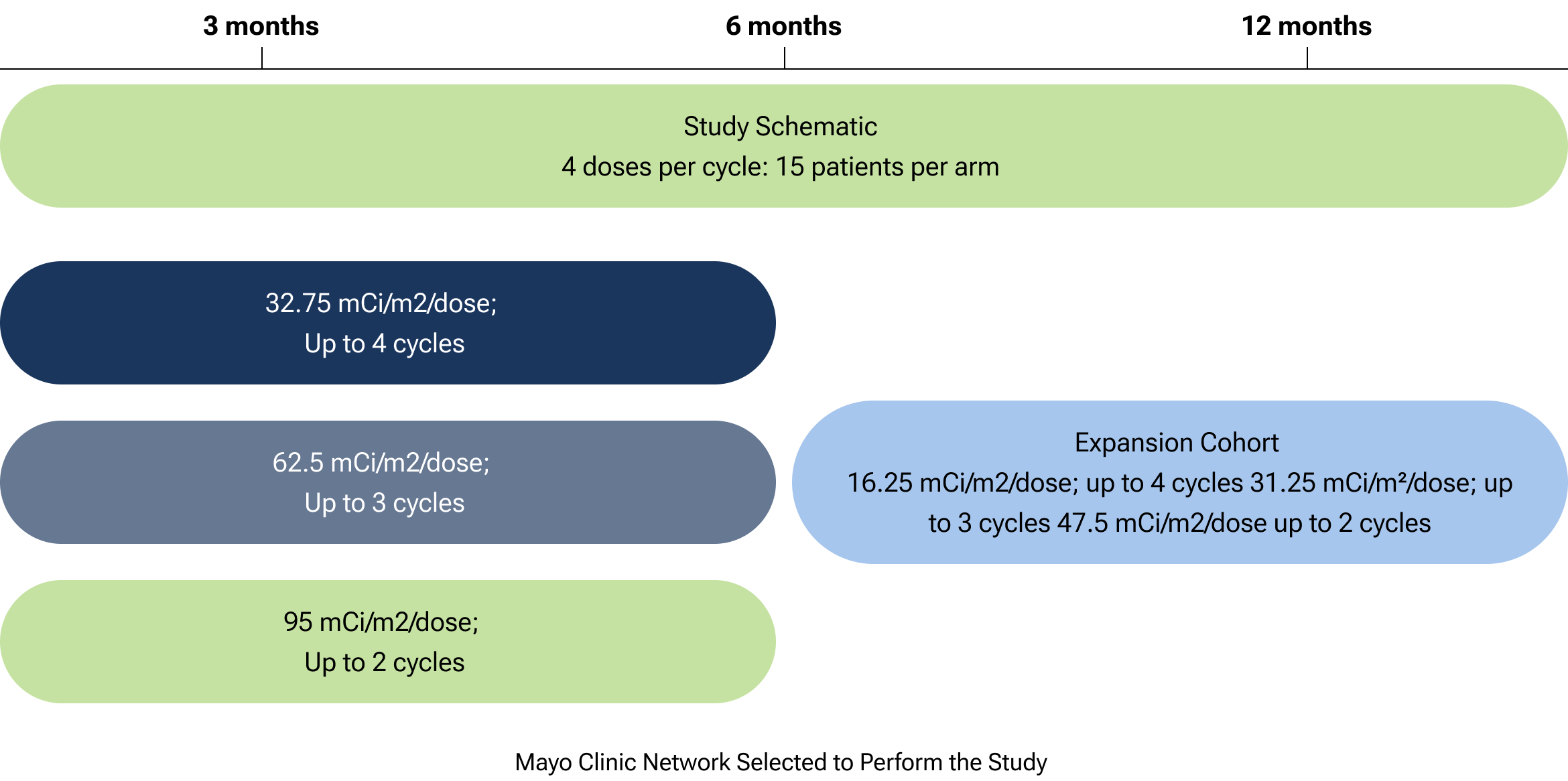
The graphic outlines the Phase 1b dose-finding study of CLR 125 in relapsed triple-negative breast cancer. It includes 15 patients per arm receiving two doses per cycle. Dose levels progress from 32.75mCi/m² (up to 4 cycles) to 62.5 mCi/m² (up to 3 cycles) and 95 mCi/m² (up to 2 cycles). The design uses accelerated intrapatient escalation followed by a standard 3 + 3 method to determine the recommended Phase 2 dose, with safety and tolerability as key endpoints. The study is being conducted within the Mayo Clinic Network.