CLR 121125 (CLR 125): Auger Emitter (125I)
A next-generation investigational phospholipid ether (PLE) conjugate designed for highly localized radiation delivery through Auger electron emission.
A next-generation investigational phospholipid ether (PLE) conjugate designed for highly localized radiation delivery through Auger electron emission.
CLR 125 is an investigational radioconjugate designed to deliver iodine-125 directly into tumor cell nucleus using Cellectar’s proprietary phospholipid ether (PLE) platform.
Iodine-125 emits Auger electrons—high-energy, short-range particles capable of inducing clustered/larger DNA double-strand breaks within targeted tumor cells while essentially eliminating any dose to surrounding healthy tissue.
Auger emitters release multiple low-energy electrons that travel a few nanometers within cells, creating dense, localized energy deposits that disrupt tumor DNA.
CLR 125’s selective uptake enables potent, targeted damage with limited off target effects.
| Name | Composition | Primary Mechanism of Cell Death | Penetrating Power (Emission Distance) | Relative Biologic Effect |
|---|---|---|---|---|
| Alpha Particles |
2 protons |
Double strand |
50 – 100um |
~5 |
| Beta Particles |
1 electron |
Single strand |
12mm |
1 |
| Auger Electrons |
Multiple electrons |
Double strand |
2 – 500nm |
1 – 5 |
Properties of different emitters provide enhanced outcomes
In preclinical studies, CLR 125 showed selective tumor uptake and statistically significant activity in animal models of triple-negative breast cancer (TNBC), with no observed end-organ or hematologic toxicity at evaluated doses.
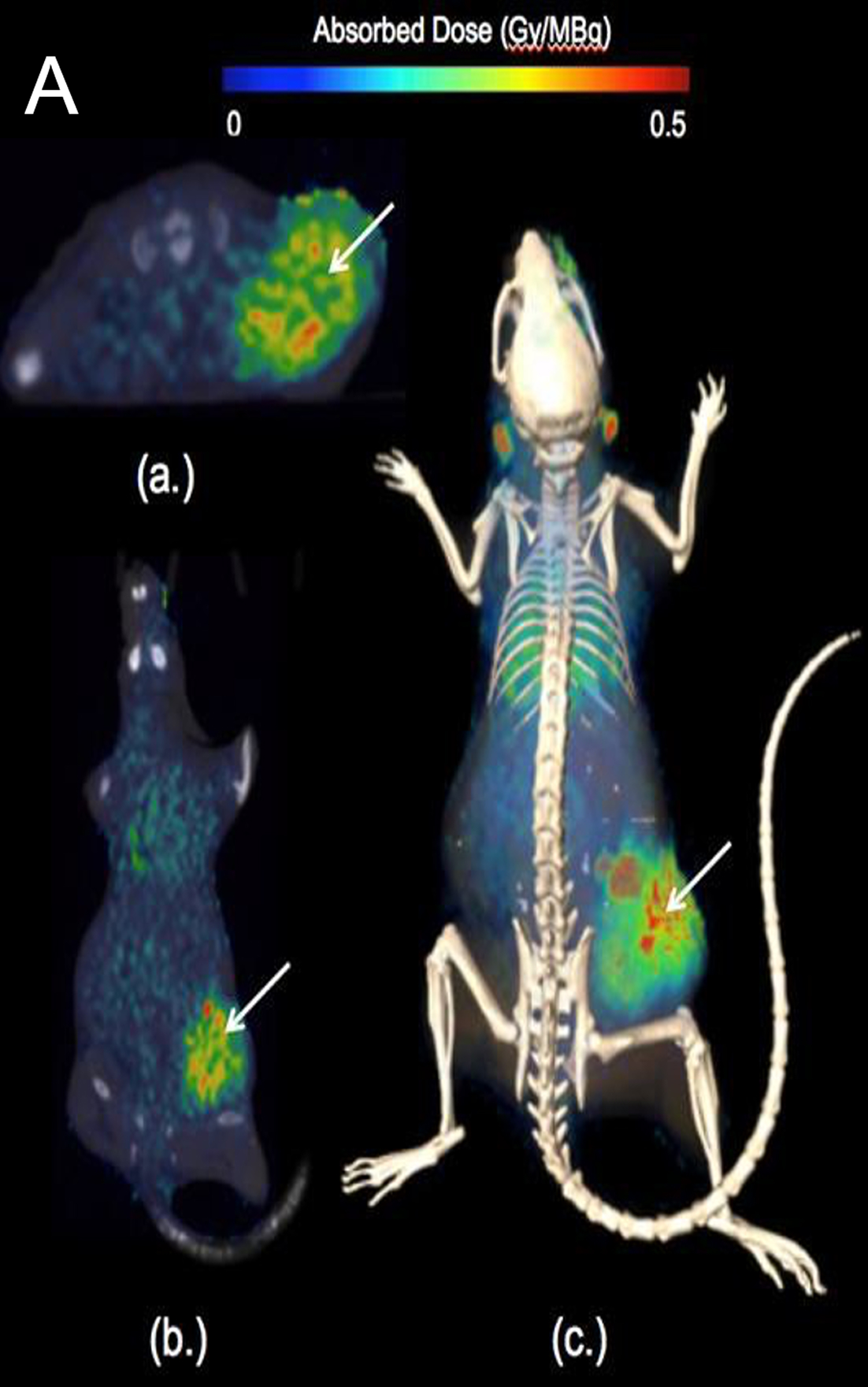


Validated intracellular delivery with CLR 121125
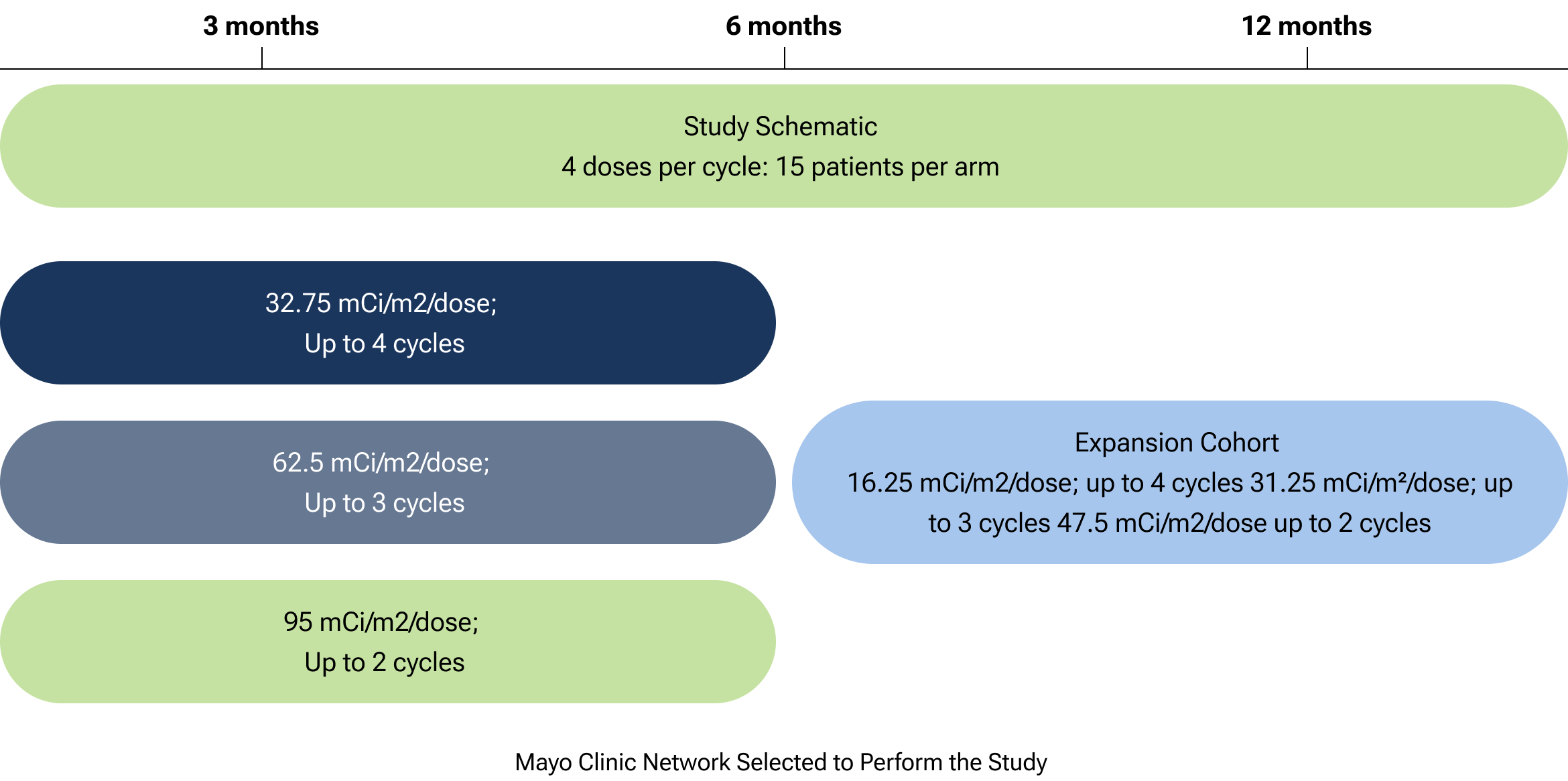
CLR 125 is currently being studied in a Phase 1b imaging and therapy trial for patients with relapsed or refractory triple-negative breast cancer (TNBC). The study, a multi-center study including the Mayo Clinic Network, aims to determine the recommended Phase 2 dose and evaluate safety and tolerability.
CLR 125 represents the continued evolution of Cellectar’s PLE platform, extending its precision-targeting approach across multiple isotope classes—beta (I-131), alpha (Ac-225), and Auger (I-125).